2.1 Bonding and Lattices
In the introductory chapter Minerals and Rocks, you learned that geologists define a mineral as being a naturally occurring, solid material made of an orderly crystalline structure that is represented by a defined chemical formula, and is generally inorganic.
Practice Exercise 2.1
Using the criteria established above, which of the following materials are minerals?
- Amethyst
- Sugar
- Cubic zirconia
- Halite
- Ice
- Obsidian
See Appendix 2 for Practice Exercise 2.1 answers.
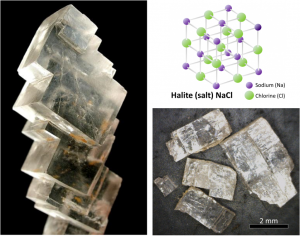
As described in the introductory chapter, all minerals are characterized by a specific three-dimensional pattern known as a lattice or crystal structure. These structures range from the simple cubic pattern of halite (NaCl) (Figure I4), to the very complex patterns of some silicate minerals. Two minerals may have the same composition, but very different crystal structures and properties. Graphite and diamond, for example, are both composed only of carbon, but while diamond is the hardest substance known, graphite is softer than paper. Their lattice structures are compared in Figure 2.1.1.

Depending on the mineral and its defined chemical formula, the bonds between the atoms the form the lattice may be ionic or covalent. On a molecular level, slight differences in the type of chemical bonds can have a significant impact on physical properties of the mineral. For example, the minerals diamond and graphite both have the same composition: both are composed exclusively of carbon atoms. In both cases, the crystal lattice is made of carbon atoms sharing electrons to form covalent bonds. In the mineral diamond, the carbon atoms are linked together in a three-dimensional framework, where each carbon atom is bonded to four other carbon atoms and every bond is a very strong covalent bond. In the mineral graphite, the carbon atoms are linked together in sheets or layers (Figure 2.1.1), and each carbon atom is covalently bonded to three others. Graphite-based compounds, which are strong because of the strong intra-layer covalent bonding, are used in high-end sports equipment such as ultralight racing bicycles. Graphite itself is soft because the bonding between these layers is relatively weak, and it is used in a variety of applications, including lubricants and pencils.
Mineral lattices have important implications for mineral properties, as exemplified by the hardness of diamond and the softness of graphite. Lattices also determine the shape that mineral crystals grow in and how they break. For example, the right angles in the lattice of the mineral halite influence both the shape of its crystals (cubic), and the way those crystals break (Figure 2.1.2).
Media Attributions
- Figures 2.1.1, 2.1.2 (top and bottom right): © Steven Earle. CC BY.
- Figure 2.1.2 (left): Halite. © Rob Lavinsky, iRocks.com. CC BY-SA.
The regular and repeating three-dimensional structure of a mineral.
A bond in which electrons are transferred from one atom to another, thus forming ions.
A bond between two atoms in which electrons are shared.

