Acetylation
A conjugation reaction carried out on drugs containing an amenable nitrogen atom by enzymes called N-acetyl transferases, predominantly N-acetyltransferase type 2 (NAT-2) in the liver and GI tract. An acetyl group is transferred from the carrier acetyl-CoA to a nitrogen on an amine, sulfonamide or hydrazine group. The acetylated metabolite is usually inactive, or has lost most of the activity of the parent drug.
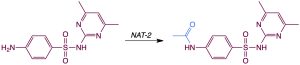
N-Acetyltransferase enzymes are more usually involved in leukotriene biosynthesis but they can accept some drugs as substrates and acetylate them. The N-acetyl metabolites of some drugs – such as the sulfonamide antibacterials – may be less water-soluble than the parent drugs, and this can cause metabolites to precipitate in the kidneys as they are concentrated in the renal filtrate. This problem is more prevalent in so-called “fast acetylators”, a portion (around 50%) of the population that have high N-acetyl transferase activity. Due to a genetic polymorphism, the other 50% of the population are “slow acetylators” and precipitation of metabolites is less problematic.

