Ecological Responses
Bioclimatic Envelope Models
The most common approach for predicting species responses to climate change is bioclimatic envelope modelling (Araujo and Peterson 2012; Gray and Hamann 2013). In this approach, a statistical model is used to characterize the climatic conditions within the current range of a species (i.e., its climate envelope or space). Once the relationship between climate and range has been quantified, predictions can be made about where the species is likely to be located in future periods, after climatic conditions have changed. The underlying assumption, supported by the paleoecological record, is that a species will track its preferred climate as it shifts through space and time (Martinez-Meyer et al. 2004; Wiens et al. 2010). The same basic approach can be used to predict spatial shifts of entire biomes (Hamann and Wang 2006; Rehfeldt et al. 2012).
One use of bioclimatic modelling is to estimate the climate “exposure” of a species. Exposure is a combination of the amount of expected change in the climate envelope and the velocity of change, which is the distance that a climate envelope shifts per unit of time (Loarie et al. 2009; Hamann et al. 2015). Faster rates of change present a greater risk because there are limits to how fast a species can shift its range.
Climate sensitivity is a complementary measure that describes a given species’ ability to cope with change. It is a function of species-specific adaptability traits. Anthropogenic disturbances that reduce resilience or create barriers to movement are also a factor. Climate exposure and climate sensitivity are combined to produce an overall vulnerability assessment.
Bioclimatic projections are also used to support a variety of planning applications, which we will discuss later in the chapter. These include:
- Identifying areas of relative stability that can serve as climate refugia
- Guiding restoration programs
- Designing protected area systems and connectivity networks
- Facilitating the range shifts of individual species
Bioclimatic envelope models are subject to several limitations that conservation practitioners should be aware of (Pearson and Dawson 2003). Foremost, model projections represent the state of the system after biotic elements have had time to equilibrate with the climate. Insight into transitional stages, which will predominate during this century, must be obtained separately through empirical study.
Another limitation of bioclimatic envelope models is that their reliability declines rapidly when applied below the regional scale. This is because factors other than climate, such as soil type, topography, disturbance history, and biotic interactions become increasingly influential in determining ecological patterns as one moves from the regional to the local scale. Furthermore, global climate models do not provide the resolution needed for fine-scale applications.
Bioclimatic envelope models are also subject to all of the limitations of statistical habitat models that we discussed in Chapter 6. In particular, the application of these models to future periods represents an extrapolation to novel conditions, making reliability difficult to judge (Pearson and Dawson 2003).
The paleoecological record indicates that the relationship between climate and ecological distributions is reasonably robust, though not inviolable (Martinez-Meyer et al. 2004). Major ecosystem types appear to have shifted northward largely intact during the Hypsithermal period (approximately 6,000 years ago), when temperatures in Canada increased by 2–3°C (Dyke 2005; Strong and Hills 2005). This suggests that bioclimatic envelope models should be reliable under similar levels of warming. But existing bioclimatic relationships may well break down under higher levels of warming, as may occur under median- to high-emission scenarios. In short, the warmer it becomes, the more uncertainty there is about how ecological systems will respond.
Vegetation Responses
Bioclimatic envelope model projections indicate that species and ecosystems in Canada will shift significantly northward and upslope under even the lowest CO2 emission scenarios (Hamann and Wang 2006; Gray and Hamann 2013). The exact amount of ecological change will vary by region and will depend on how much warming actually occurs. Changes will be most apparent along ecotones and in parts of the country subject to both warming and drying.
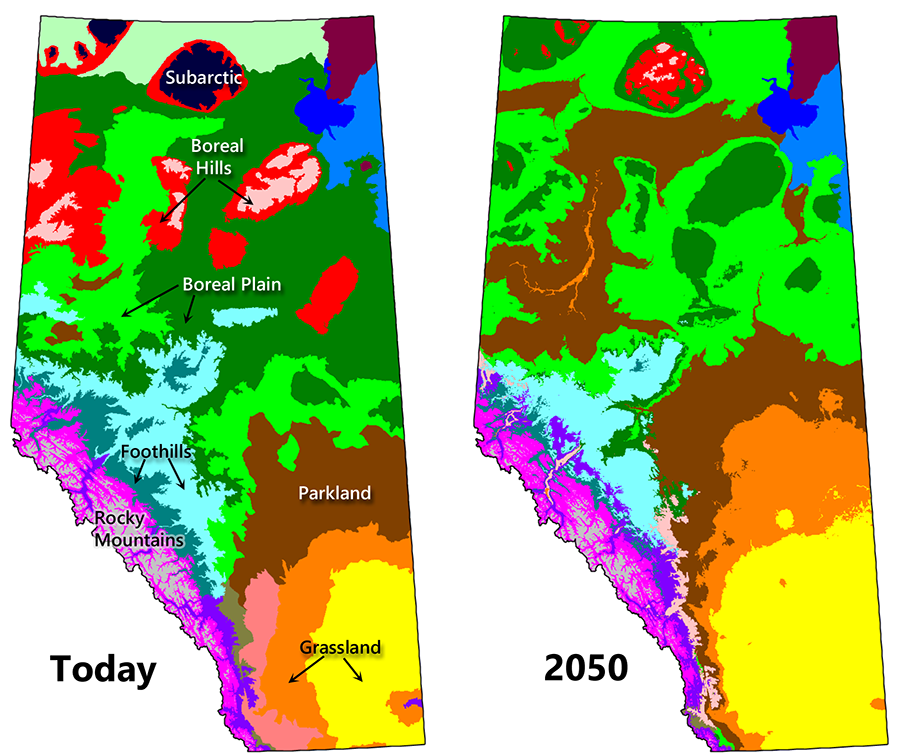
The Prairie Provinces and the Territories are expected to experience the greatest ecological shifts. A case in point is northern Alberta, which receives just enough moisture to support a closed canopy forest (Hogg and Bernier 2005). Increased evapotranspiration from higher temperatures is expected to cause a net decline in moisture levels, leading to the eventual transition of most of Alberta’s boreal forest to parkland and grassland (Fig. 9.4; Stralberg et al. 2018). Changes will be much less extreme in BC and Eastern Canada because precipitation inputs are higher, making it unlikely that forests will become moisture stressed under warmer conditions (Price et al. 2013). In fact, these forests are expected to become more productive and more diverse in the future (D’Orangeville et al. 2016). Grassland regions will experience a change in species composition but are expected to remain as grassland ecosystems.
In addition to knowing the long-term trajectory of change, it is important to understand the mechanisms by which the ecological transitions will occur. Computer modelling is not yet an option, though dynamic vegetation models that include climate change are under development (Fisher et al. 2015a). For now, we must draw directly on empirical research and ecological first principles to gain insight into transitional processes.
Plants have a certain amount of resilience to climatic changes, both at the individual level, through structural and functional plasticity, and at the population level, through genetic variability (Hof et al. 2011; Reed et al. 2011). However, these mechanisms will not be sufficient to accommodate the large rise in temperature that is anticipated. Plant species will also need to shift their ranges (Corlett and Westcott 2013; Savage and Vellend 2015).
Climate-induced range shifts will occur through differential reproduction and mortality along range margins. Where climatic conditions are improving for a given species, referred to as the leading edge, competitive ability will be enhanced and mortality will be reduced (Koen et al. 2014b). Over time, this will lead to increased abundance of the species along the leading edge, and the colonization of new landscapes ahead of it. Similar processes will occur in reverse along the trailing edge of the range, leading to lower abundance and abandonment of range (though not necessarily at the same rate). The net effect is a slow directional shift in range.
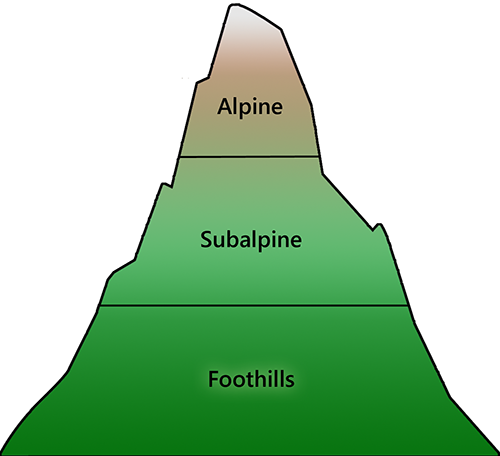
It is important to note that climatic effects will not be limited to changes at range margins. Warming will disrupt the competitive balance that exists among species in all areas (HilleRisLambers et al. 2013; Hargreaves et al. 2014). From paleoecological data and contemporary ecological studies (Martinez-Meyer et al. 2004; Wiens et al. 2010), we know that the natural balance among species will seek to re-establish itself over time, as competitive interactions exert their inexorable effect (subject to caveats discussed below). Consequently, an observer in a fixed location will see a gradual change in the relative abundance of species over time as this rebalancing takes place (Kelly and Goulden 2008). At the macro scale, these changes will manifest as a slow directional shift in the distribution of entire plant communities along the prevailing climatic gradient (Fig. 9.5).
 |
 |
 |
|
 |
|
| Fig. 9.5. The influence of climatic gradients on ecological distributions are easiest to observe in mountainous areas, where changes occur across relatively short distances. Moving upslope, mixed coniferous and deciduous forests give way to a sequence of coniferous forest types, and eventually, to alpine meadows that are largely devoid of trees. The elevation at which these transitions occur depends on latitude, indicating that it is mainly temperature, not elevation, that is responsible for the observed pattern. Photo credits: Foothills: J. Pang; Subalpine: P. Krömer; Alpine: D. Hershman. | |
As a general rule, ecological transitions will lag significantly behind changes in the climate. Ecological systems have inertia, much of it due to the “priority of place” (Suttle et al. 2007; Hille- RisLambers et al. 2013). Established plants are not easily displaced by new arrivals, even if the climate has become suboptimal for them (Urban et al. 2012). Superior competitors will eventually prevail but they may need to wait for a window of opportunity provided by the mortality of existing plants. This may take decades in forest systems.
Aspen provides a useful example. Recent surveys along the eastern slopes of the Rocky Mountains have found aspen seedlings growing as high as 1,500 m, which is 200 m higher than previously recorded (Landhäusser et al. 2010). All of these seedlings were growing within forestry cutblocks, which provided the disturbed conditions needed for aspen establishment. Aspen was unable to establish within adjacent mature forest stands, even though climatic conditions were similar. The implication is that the potential range of aspen has already expanded as a result of the warming that has occurred in recent decades. However, the utilization of this new range is being impeded by the presence of existing vegetation. Most species are likely to experience these sorts of lag effects (Bedford et al. 2012; Gray and Hamann 2013).

The aspen example demonstrates that disturbance rates will be a key factor governing the rate of ecological transitions. Anything that kills or seriously weakens existing vegetation, including fire, severe drought, insect outbreaks, or windthrow, will provide a window of opportunity for competitive rebalancing and range shifts. Conversely, areas that remain undisturbed will transition much more slowly, despite progressive changes in climate. As a result, instead of progressing as a wavefront, ecological transitions will be patchy and widely distributed, reflecting the scattered distribution of natural disturbances (Fig. 9.6). The types of transitions that occur within disturbed sites will depend on how much climatic warming has occurred to that point and on local factors such as the availability of seed sources.
Another factor that limits vegetation responses to climate change is dispersal ability (Urban et al. 2012). The rate that plants can invade new landscapes, given suitable conditions, depends on the distance their seeds can travel and the time it takes for seedlings to mature and produce seed. Given the extraordinarily fast rate of climate warming, few species will be able to disperse fast enough to keep pace with the leading edge of their climatic envelope (Corlett and Westcott 2013). The ones that do are likely to be pioneer species, like fireweed, with adaptations for exploiting new and potentially distant habitats. A corollary is that climate change is expected to facilitate the expansion of invasive species, both native and alien (Walther et al. 2009).
Because species have different rates of dispersal, ecosystems will not shift as intact units. Instead, we can expect novel combinations of species to arise during the transitional period, as some species race ahead and others lag behind (Urban et al. 2012). The faster the rate of warming, the greater these anomalies will be.
Changes in ecosystem composition are also expected to arise from differences in species resilience. For example, feedback processes within peatlands provide resilience to warming and drying (Waddington et al. 2015). As a result, large peatlands will likely persist in the western boreal forest well into the next century, despite increasing moisture deficits (Schneider et al. 2016). In contrast, white spruce is expected to steadily decline as a consequence of regeneration failure under drier conditions. The resulting transitional ecosystem, dominated by peatlands, aspen, and open grassland, will be unlike anything that currently exists in Canada today.
In summary, we can expect that ecosystems will generally follow the trajectories described by bioclimatic envelope models, but the projected equilibrium conditions will not be reached until well into the next century, or beyond. In the meantime, novel transitional ecosystems will predominate. The rate and spatial pattern of vegetation transitions will depend on both the rate of warming and the occurrence of natural disturbances. Ecosystems will become complex admixtures of old and new elements, blurring ecosystem boundaries and increasing habitat diversity in most regions (Berteaux et al. 2010; Savage and Vellend 2015).
Box 9.1. An Old‐Growth Bottleneck
Old-growth forest stands are lost to natural disturbances and forest harvesting, and they are replenished through the maturation of younger stands. The rate of loss will increase under climate change because of an increase in the rate of fire and other natural disturbances (Kuuluvainen and Gauthier 2018). These losses will eventually be offset by the expansion of forests into more northerly regions. However, the rate of old-growth production is expected to be much slower than the rate of loss for many decades, resulting in a critical bottleneck. Maintaining a minimum amount of old-growth forest during this transitional period will require a reduction in harvest rate and careful spatial planning.
Animal Responses
As with plants, bioclimatic envelope models suggest that animal distributions will shift northward and upslope in response to warming temperatures, with considerable individual variability. Again, these are equilibrium projections that will not be realized immediately. Transitional processes will predominate well into the next century.
Climate affects animal distributions through a combination of direct physical effects and indirect effects on habitat supply. Habitat generalists will respond to climate change differently than habitat specialists. For generalists, the direct effects of warming are likely to be most important. For example, white-tailed deer are found across many ecosystem types, from forests to grasslands. The northern extent of their range is thought to be determined mainly by winter severity, which affects survival and reproduction (Dawe and Boutin 2016). Warmer winters are likely to lead to range expansion long before vegetative communities have responded. Evidence of such climate-induced range expansion is already accumulating (Veitch 2001; Dawe and Boutin 2016).

For most other animal species, habitat suitability is likely to be the main factor governing range shifts (Kissling et al. 2010; Nixon et al. 2016). For example, the climate envelope for the burrowing owl—an endangered grassland species at the northern extent of its range in Canada—is projected to move northward fairly rapidly (Fig. 9.7; Fisher and Bayne 2014). However, the owls will not be able to utilize this expanded potential range until entire grassland ecosystems, including appropriate vegetation and prey species, are in place. The upshot is that, despite their high mobility, many animal species will be unable to respond to climate changes any faster than the plant species they ultimately depend on (Nixon et al. 2016).
An additional problem in adapting to climate change is that many species must simultaneously cope with the effects of industrial development and other human activities. The more a species is impacted by human disturbances, the lower its adaptive capacity for responding to climate change.
The concern is greatest for species that are struggling to remain viable under current conditions. Returning to our burrowing owl example, instead of being primed to expand along the northern leading edge of its range, as conditions become suitable, it is currently declining in abundance and contracting southward toward the core of its range in the US (COSEWIC 2006). Moreover, its small remnant populations in Canada may lack the resilience needed to withstand extreme weather events, which are expected to become more common as the climate warms (Oliver et al. 2013; Fisher et al. 2015b).
Human disturbances can also form physical barriers that impede a species’ ability to track its preferred climatic conditions. Habitat fragmentation presents the most widespread barrier, and while not completely blocking movement, it slows the pace of adaptation (Barber et al. 2016). For example, the ability of a grassland species to track its preferred climate as it moves northward is likely to be hindered by the presence of intensively managed agricultural land (Nixon et al. 2016). For fish, stream fragmentation may reduce their access to climatically optimal watercourses (Park et al. 2008).
Indirect Effects
Canada’s harsh climate has been a barrier for many non-native invasive species, but this barrier is now weakening. New alien species are expected to become established here, and those that are already present are expected to expand their distribution (Walther et al. 2009; Smith et al. 2012).
Another indirect effect of climatic warming involves changes in human activity patterns. Of particular significance to biodiversity is the potential for agricultural expansion into areas that are currently forested. As we saw in Chapter 5, agriculture is far more detrimental to biodiversity than forestry and other forms of industrial activity. Such expansion is already being contemplated. For example, in their analysis of climate change implications for Saskatchewan, Carr et al. (2004) write:
As areas in southern Saskatchewan and Alberta become too arid for maintenance of herds of animals, it may be possible to open up new agricultural areas north of Prince Albert. These areas are too cold now, but as the length of the growing season increases, perhaps the present boreal plain can be made the new breadbasket of the country. (p. 26)
Although agricultural expansion would provide important societal benefits, including jobs and food production, it nevertheless represents a serious threat to the conservation of native biodiversity. This is especially true if the transition to agriculture involves the privatization of public land, and concomitant loss of government control over land-use practices.
A related threat to biodiversity is that, as the climate warms, resource companies may demand a loosening of operating rules designed to maintain biodiversity (Carr et al. 2004). Companies may argue that such restrictions are futile—like building sandcastles before the tide—given the impending overriding effects of climate change. But such arguments disregard the importance of supporting species as they adapt to change. In the face of climate change, species need more help, not less.
Change Versus Threat
Though widespread changes in ecological distributions seem inevitable, these changes need to be placed in context. Not all changes are threats. Much depends on the frame of reference used.
Species diversity in Canada declines with latitude, and overall it is relatively low compared with many other countries (Willig et al. 2003). A northward movement of ecosystems under climate change would boost biotic productivity and species richness at all latitudes (Jia et al. 2009; Savage and Vellend 2015). Thus, from the perspective of species richness, climate change may be a generally positive factor for Canadian biodiversity (Berteaux et al. 2010), though there are caveats that we will discuss below.
If we use regional ecosystems as our frame of reference, it is apparent that a warmer climate will result in both “winners” and “losers” (Zhang et al. 2015). For example, alpine and high Arctic ecosystems will be compressed because there is nowhere for these ecosystems to go. Conversely, grassland ecosystems in the Prairie provinces, and mixedwood systems in the Niagara and St. Lawrence regions, are expected to expand into the southern boreal region, making them beneficiaries of a warming climate (Schneider et al. 2016). Net changes in other ecosystems are difficult to predict because they will experience both gains and losses along their leading and trailing edges, respectively. Overall, it is a zero-sum game: losses in one ecosystem must be offset by gains in another. What is notable is that the majority of Canada’s species at risk reside in southern ecosystems that are expected to expand.
During the transitional period that will predominate during this century, ecosystem complexity at the regional scale will generally be increased relative to current levels (Savage and Vellend 2015). As previously discussed, the composition and structure of existing ecosystems will initially be augmented by scattered patches of new elements. In later stages, added complexity will arise from scattered remnants of old ecosystems that are left behind as ecosystems move northward and upslope.
Turning finally to species, the level of threat posed by climate change hinges on the balance between adaptive capacity and the amount of anticipated warming. Several insights into this dynamic can be gleaned from the Holocene paleoecological record (Moritz and Agudo 2013). Canadian species are all Ice Age survivors, having experienced a 4–5°C change in global temperature during the Holocene (Clark et al. 2016). The paleoecological record shows that all of our species, including those with low apparent dispersal ability, were able to shift ranges across vast distances. In some cases, Canadian species took refuge as far south as the southern US (Pielou 1991). It is also apparent that all current species, including habitat specialists and endemic species, have the flexibility to persist under novel conditions. Glacial refugia were not replicas of current ranges. They were composed of different species assemblages, and there were differences in photoperiod, soils, and other site-related factors (Pielou 1991).
An important feature of the present episode of warming is that the rate of change appears to be faster than in previous episodes. Many species may be unable to adjust their range quickly enough to track their preferred conditions, subjecting them to climatic disequilibrium (Malcolm et al. 2002; Corlett and Westcott 2013). The ability of species to persist in a state of climate disequilibrium is currently a topic of active debate. Some researchers assert that climate envelope fidelity is necessary for species persistence, and their predictions concerning species viability are often dire. For example, a highly cited paper by Thomas et al. (2004) predicts that 15–37% of global endemic species will go extinct because their current climate envelope will disappear or become inaccessible to them.
The counterargument is that climatic disequilibrium is not a novel phenomenon. It was also experienced during the Ice Age transitions. Global temperatures had already risen to near current levels by the beginning of the Holocene (11,700 years ago; Clark 2016), but returning species could not immediately establish themselves in their current ranges. Much of Canada was still under ice at that time, and freshly exposed landscapes lacked soil (Pielou 1991). Despite the climatic and ecological disequilibrium that species must have experienced during this period, extinction rates did not increase (McInerney and Wing 2011; Willis and MacDonald 2011). Based on accumulating paleoecological evidence, some researchers now believe that species have greater climatic flexibility than has previously been assumed (Pearson 2006; Hof et al. 2011).
Even if climate envelope fidelity is not an absolute requirement for persistence, there undoubtedly are limits to how much accommodation is possible. We do not know what the thresholds are, but the greater the degree of disequilibrium, and the longer it lasts, the higher the likelihood that some species will decline or become extirpated as a result. It is also evident that certain species traits increase vulnerability (Pearson et al. 2014):
- Narrow physiological tolerance limits
- High degree of habitat specialization
- Small range size
- Low dispersal ability
- Low rate of reproduction
- Low genetic variation within and among populations
- Sensitivity to human disturbance
A caveat when using Ice Age comparisons is that the Quaternary featured a change from cold to warm, whereas we are now changing from warm to hot. This has particular relevance for cold-adapted species found in high alpine areas and along the Arctic coast. These species are likely to be confronted with conditions that are novel to them, and which cannot be accommodated through range shifts. Pushed toward mountaintops and the sea, some of these species may find themselves trapped—physically unable to shift to cooler sites and outcompeted by species adapted to warmer climates.
Another important caveat is that past warming episodes occurred in the absence of an anthropogenic environmental footprint. More than anything else, it is the synergy between environmental degradation and climate change that raises the spectre of widespread species extinction (Hof et al. 2011). Species that are sensitive to human disturbance have experienced range contractions and declines in abundance and are generally less resilient to change of any type. For those species currently struggling to remain viable, the added stress imposed by rapidly changing climatic conditions may be what ultimately seals their fate. More generally, physical barriers and habitat deterioration can reduce adaptive capacity by hindering species movements and range adjustments (Nixon et al. 2016).
In summary, climate change will have both positive and negative influences on biodiversity. Some species and ecosystems will undoubtedly decline, as their climate envelopes contract. But overall, the moderating of Canada’s harsh climate, together with complex transitional dynamics, will promote an increase in diversity across the country. The caveat is that these positive outcomes may not be realized if human land uses preclude effective adaptation. The proportion of species “left behind” may be relatively small under low-end warming scenarios, assuming that management efforts are made to reduce barriers to movement and to actively assist highly vulnerable species. But widespread adaptation failure is a real possibility under high-end warming scenarios, which take us into uncharted territory.

