7 Water
This chapter is a near-final draft. Glossary entries, and some figures remain to be fixed.
In this chapter we introduce the Hydrosphere, with a look at its major component, water.
Learning Outcomes
- Describe the water molecule, explain why it is polarized, and the significance of that polarization
- Describe the physical properties of water during melting and freezing
- Explain the chemical behaviour of water as it dissolves other substances
- Review the hydrologic cycle, hydrologic reservoirs, and water fluxes
Water molecules
Electric charge in water molecules
Water molecules are formed by covalent bonding of two hydrogen atoms to an oxygen atom. The bonds are mainly covalent, meaning that electrons are shared between the atoms so that their electron shells contain a stable number of electrons. Each hydrogen atom ends up with two electrons, while the oxygen atoms has eight. Because of the behaviour of electron orbitals, the molecule has a “bent” shape with an angle of 104.5° between the two bonds.
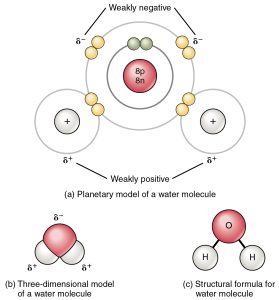
However, the bonds are not completely covalent: the electrons can be thought of as spending more time near the oxygen atom than near the two hydrogen atoms. As a result the molecule is polar: there is more negative charge near the oxygen atom, and more positive near the two hydrogen atoms. As we shall see, many of the unusual properties of water result from this polar character.

For example, water molecules tend to develop weak hydrogen bonds with other polar molecules, including other water molecules. This contributes to the unusual properties of ice, which is less dense than liquid water. Hydrogen bonds between liquid water molecules give water its strong surface tension, allowing it to form nearly spherical droplets, and allowing some insects to support themselves on a water surface despite being denser than water.

The polar character of water makes it an excellent solvent, particularly for ionically bonded and other polar substances, including many minerals.
Dissociation of water molecules: pH

Because of the polar behaviour of electrons in the bonds within water molecules, occasionally the bonding electrons will migrate all the way to the oxygen atom, causing a hydrogen ion H+ to break free, leaving behind a negatively charged hydroxyl ion OH–. As written, a hydrogen ion would consist of an isolated proton; in actuality free protons don’t exist in water: they are immediately attached to the negatively charged oxygen end of a nearby water molecule producing a hydronium ion H3O+. (When chemists speak of hydrogen ions, they typically are using a shorthand for hydronium ions.)
The overall reaction can be written
2H2O ⇋ H3O+ + OH–
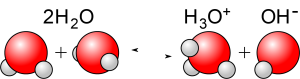

The concentration of hydrogen (or hydronium) ions in natural water is very small. In pure water, only one molecule in ten million (107) is dissociated, so the concentration of hydrogen ions is 10–7. However, the concentration of hydrogen ions can be changed by adding dissolved mineral materials or atmospheric gases to the water. Water with more hydrogen ions than pure water is said to be acid. If the concentration of hydrogen ions is even lower than that of pure water, the water is said to be alkaline or basic.
The acidity or alkalinity of water is measured by the quantity pH, defined as minus the log (to base 10) of the hydrogen ion concentration. This means that neutral water has a pH of 7. If the concentration of hydrogen ions is increased to 10-6 (acid) then the pH is 6. Conversely if the concentration of hydrogen ions is reduced to 10-8 then the pH of the alkaline water is 8.
The diagram shows typical pH values for natural and artificially occurring waters.
Typical ocean water is slightly alkaline (pH = 8 to 8.5) whereas some surface waters are quite acidic (pH 4 to 5).
Most rainwater is slightly acidic too (pH around 5.6). To understand why, we need to look at how the atmospheric gas carbon dioxide behaves when it dissolves in water.
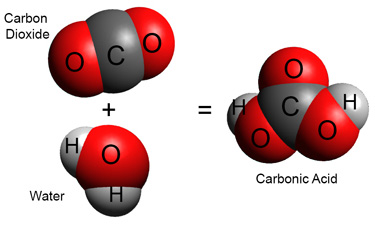
Carbon dioxide dissolves in water to make molecular carbonic acid.
However, most of this carbonic acid loses a hydrogen ion right awayto form a bicarbonate ion HCO3–
H2CO3 = HCO3– + H+
The hydrogen ions immediately associate with water forming hydronium ions. They lower the pH, making the rainwater slightly acid.[1]![]()
 |
 |
 |
Phases of water
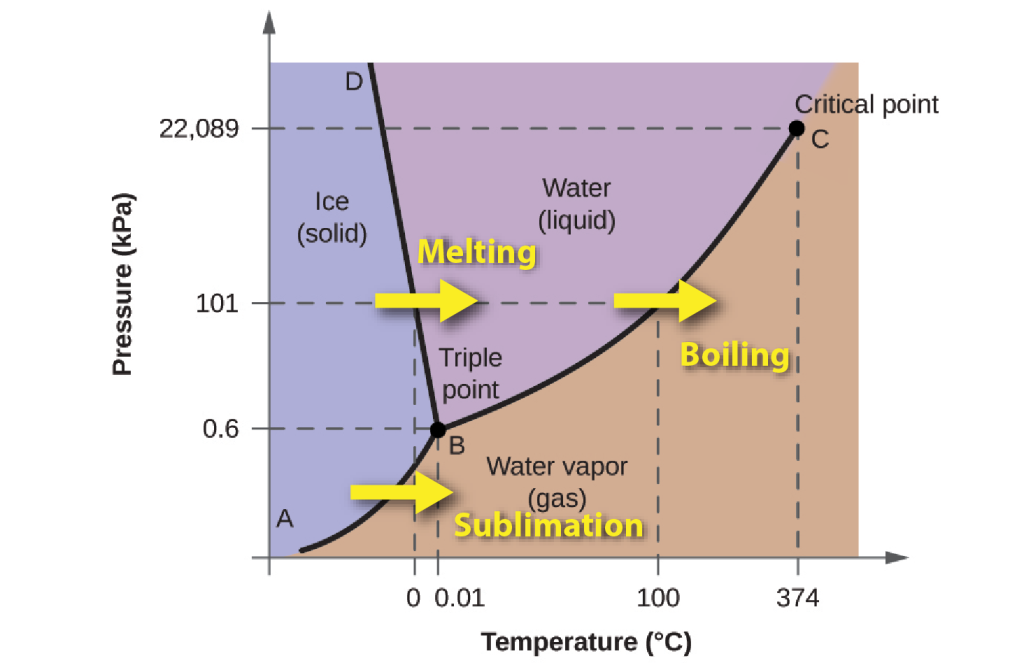
Water can exist in three different forms known as phases: solid ice,liquid water, and water vapour Phases are parts of a system that are separated by distinct boundaries from other parts of the same system, without mixing.
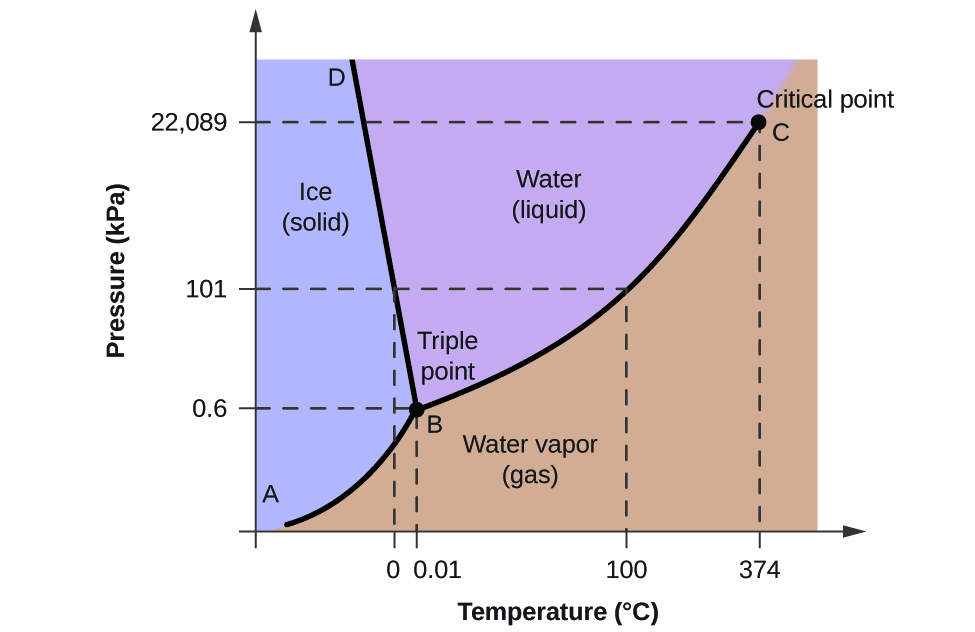
The transitions from one phase to another are controlled not only by temperature but also by pressure, so to illustrate the behaviour of water we use a phase diagram, with temperature and pressure along the axes. The phase diagram shows which form of water is stable at any combination of temperature and pressure. For example, at a pressure of 101 kPa (one atmosphere) ice melts at 0°C and liquid water evaporates at 100°C. There’s one set of conditions (0.6 kPa and 0.01°C) where all three phases can exist in equilibrium together: it’s known as the triple point for water.
Ice

The solid form of water, ice, is a crystalline substance. That means that the water molecules are arranged in a regular repeating crystal lattice. The 6-fold symmetry of this internal structure is responsible for the beautiful symmetry of snowflakes:- ice crystals that are formed by direct condensation of water vapour into ice.
The crystal structure of ice has a lot of open space, with the result that ice is less dense than liquid water, so that ice floats. This is an unusual physical property. Most substances expand when they melt and contract when they freeze. For example, when molten rock (magma) freezes to form minerals, those minerals are usually denser than the magma and sink to the bottom of the magma. Water is an exception. It actually expands as the temperature gets colder. From 4°C down to a temperature of about -20°C, water and ice expand as they get colder. The total expansion is about 9%. (This is also why ice floats: because of the expansion, ice is less dense than water.)
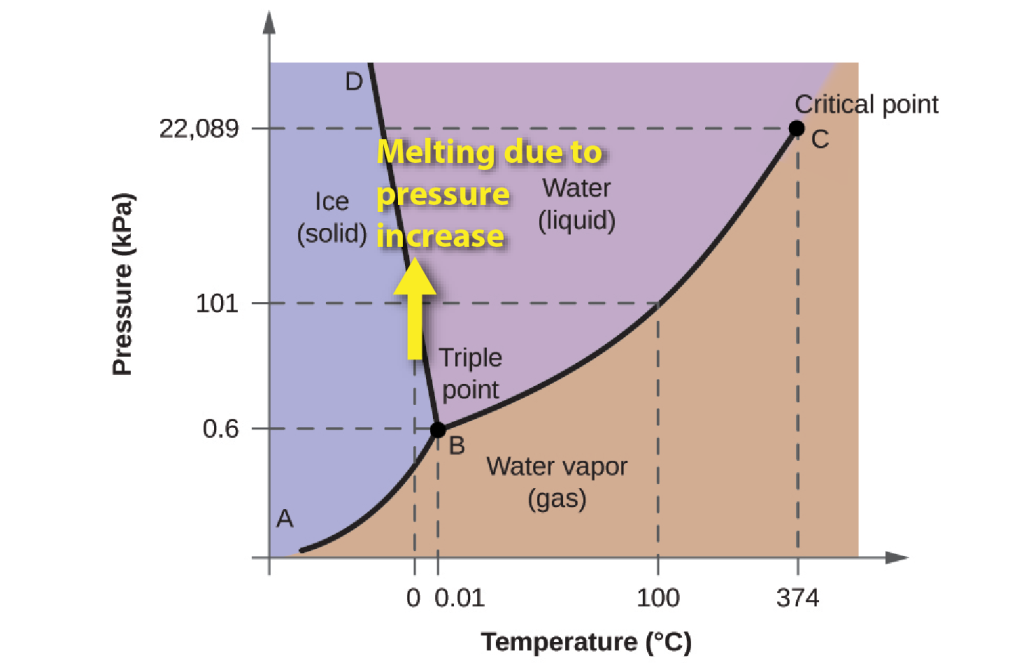
Because of this property, the boundary between ice and water on the phase diagram has a negative slope. As the pressure increases, the melting point of ice falls. This means that when ice is subjected to increased pressure (for example, beneath a glacier or beneath an ice-skate) it may melt without changing temperature.
Because of the regular crystal structure of ice, it’s not able to hold many other ions in solution. When ice is formed by freezing of ocean waters, the dissolved salt stays in the remaining ocean water.
Liquid water
Liquid water has a disordered structure, in contrast to the regular crystal lattice of ice, but the molecules are still held together by hydrogen bonds. Because of its disordered structure, and polar character, liquid water can easily accommodate dissolved ions.

Dissolved ions account for the saltiness of sea-water, which contains about 3.5% of dissolved solids. The dissolved ions actually make liquid water more stable, by hindering the process of ice formation. Hence seawater typically freezes at a temperature about 2°C lower than fresh water, and a saturated brine, containing 23.3% by weight of salt, doesn’t freeze until it reaches -21°C.
Water vapour
Water vapour exists in varying concentrations in the atmosphere, up to about 4%. The amount of water vapour that can be dissolved in the atmosphere in equilibrium varies with temperature, as is described in the chapters on the Atmosphere.
In contrast to liquid water, which is almost incompressible, the density of water vapour increases as pressure rises. At high pressures, above 22 MPa, and temperatures above 374°C, the liquid and vapour phases become indistinguishable, and a single supercritical fluid phase exists.
Transitions between phases: latent energy
Heating any form of water requires energy, to increase the average kinetic energy of the molecules, but crossing any phase boundary from left to right in the diagram requires extra energy, known as latent energy, which is used to separate the bonds that exist (in the case of water, hydrogen bonds) between the molecules in the solid and the liquid state. The energy is referred to as “latent” because it doesn’t change the temperature as it is added. For example, it takes about the same amount of energy to raise ice from -16°C to 0°C as it does to convert that ice to water at 0°C.
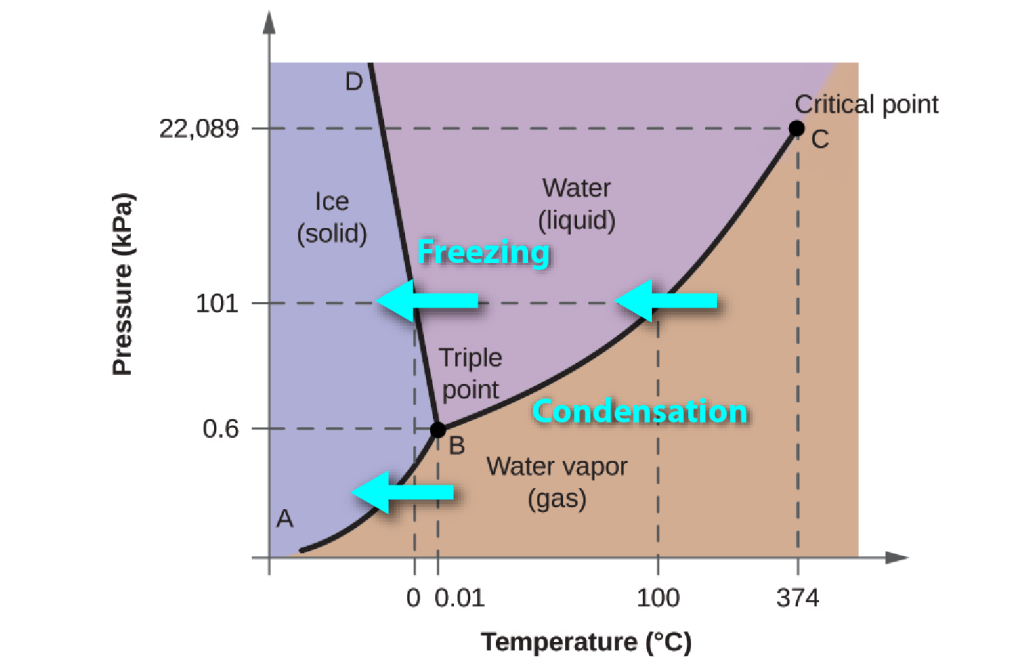
During downward changes in temperature, as liquid water is cooled to make ice, or as water vapour condenses to form liquid or ice, that latent energy must be removed in order for the phase change to be achieved.
Water and weathering
Weathering is the modification of the surface of the Geosphere as a result of the action of the Atmosphere, Hydrosphere, and Biosphere. Broadly speaking, we can distinguish physical (or mechanical) weathing from chemical weathering. Physical weathering generates fragments of rock that are chemically unchanged, whereas chemical weathing causes changes to the solid minerals and other substances of the Geosphere. Water is tremendously important in both types of weathering.
Weathering is typically accompanied by erosion: erosion is the removal of weathered material from the site of weathering. In some circumstances weathering and erosion take place almost simultaneously. Elsewhere, weathered material may sit around on the surface of the Geosphere for some time before being eroded. Weathered material that is still more or less in place is called regolith. In most parts of the world, regolith provides a fertile growing medium for plants; regolith that contains plant material is called soil.
Physical weathering by ice and water
Freeze-thaw effects

The expansion of water as it freezes to form ice has can exerting very large forces when freezing takes place in a confined space. When water penetrates into cracks and crevices in the surface of the Geosphere, and then freezes, these forces help to break rocks apart, a process known as frost action or frost wedging. Frost heaving is a related process that occurs when there are horizontal cracks; expansion of ice lifts the ground surface. Frost wedging and frost heaving are major processes of weathering in cool temperate climates where there are many freeze-thaw cycles in the course of a year.
Abrasion
Once fragments have been removed from the surface of the Geosphere they may be transported (see below) by water. Along coastlines with large waves, and in fast-flowing rivers, these fragments can travel at high speed and may cause further weathering as they are hurled at rock surfaces, a process known as abrasion.
The effects of abrasion are most spectacularly seen on wave-washed coastlines where cliffs may be undercut by weathering and erosion. Exposed headlands may turn into natural arches and sea stacks as a result of these processes.

Chemical weathering by water
Water is a powerful solvent and is able to dissolve minerals that are ionically bonded as shown earlier in this section for the simple case of halite, or rock salt (sodium chloride).

A more common solution reaction involves calcium carbonate, the main component of limestone. Solution of calcium carbonate involves hydrogen ions (or, strictly speaking, hydronium ions), so it proceeds faster if the water is acid – its pH is low.

Following the reaction, the abundance of hydrogen ions in the water is much reduced, so water that has flowed over limestone is typically slightly alkaline. The water also contains an increased concentration of bicarbonate ions, together with a new component: dissolved calcium ions.
When water that has flowed over carbonate rocks like limestone is used in domestic water supplies, the calcium ions react with soap, gradually reducing the lather that it produces neutralizing its cleaning effect. Such water is described as “hard” water, in contrast with “soft” water, typical of areas without carbonate rocks, where the domestic water is more acid and soap lather lasts longer.
Much of the Geosphere is made of silicate minerals, which are also dissolved by acidic waters, though the reactions are slower than for carbonates. They are also more complicated! The reaction below is one of the simplest, involving a potassium silicate mineral, feldspar, which is a common component of granite and other igneous rocks.
2KAlSi3O8 + 2H+ + H2O => Al2Si2O5(OH)4 + 2K+ + 4SiO2
In words, this corresponds to:
Feldspar + hydrogen ions + water => clay mineral + potassium ions + dissolved silica
The hydrogen ions and the water help to break apart the feldspar. For this reason, such reactions are known as hydrolysis (“water splitting”) reactions. The results include both dissolved material and a new mineral that incorporates water in the form of hydroxyl (OH) ions. The minerals produced by hydrolysis reactions tend to be very fine-grained and are known as “clay minerals“. The aluminum silicate produced by this reaction is the clay mineral kaolinite, which is the principal component of china clay, used for making fine ceramics.
Transportation of sediment
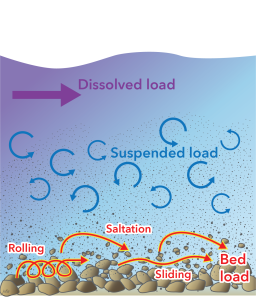
Water is important not only for weathering, but also as an agent of erosion and transportation of the material produced by weathering to new locations on the surface of the Geosphere.
There are three main ways in which water can carry material.
Bed load
Water has a property known as viscosity which causes it to exert a force on solid objects that it flows past. Because of this, water is able to slide and roll particles of rock along a river bed or along the sea-floor. Also, objects that protrude from the bed of a fast-flowing stream are subject to a lift force that tends to make rock particles jump out of the bed. They are then carried along by the flow and settle a short distance downstream, a process called saltation. The sediment carried by these three processes — rolling, sliding, and saltation, is known as bed load.
Suspended load
Most flowing water doesn’t flow in a straight line. Swirling movements called turbulence are produced by a combination of the density and viscosity of water in all but the slowest flows. Because these movements include upward flow, they are able to suspend small particles, carrying them along without contact with the bed, and making turbid (cloudy). Fine-grained sediments, including clay minerals produced by chemical weathering, are predominantly carried in this suspended load.
Because fine sediment is suspended much more easily than coarse sediment, prolonged transport by water has the effect of sorting sediment into different grain sizes, which are deposited in different places, producing well sorted accumulations of sediment in which all the grains are about the same size. For convenience, sediment is classified by grain size into three main categories:
Gravel: grains larger than 2 mm diameter. Mainly travels as bed load.
Sand: grains between 2 mm and 1/16 mm: Travels as bed-load or suspended load.
Mud[2]: grains smaller than 1/16 mm: Mainly travels as suspended load.
In contrast with liquid water, ice in a glacier shows no turbulence: the flow is laminar, meaning that the ice moves in roughly parallel lines without turbulence. However, unlike water, ice has enough strength to support large blocks or rock as well as fine-grained mud. Therefore, sediment deposited directly from ice tends to be poorly sorted — it contains material with a very wide range of grain sizes from fine-grained clay to large boulders.
Dissolved load
Many weathering processes, as we have seen, produce material in solution. This is carried by water as dissolved load. Much of the dissolved load of rivers is carried all the way to the sea, where some components are extracted by living organisms (e.g. corals, molluscs, free-floating micro-organisms) that extract ions to make skeletons of calcium carbonate (CaCO3) or silica (SiO2).
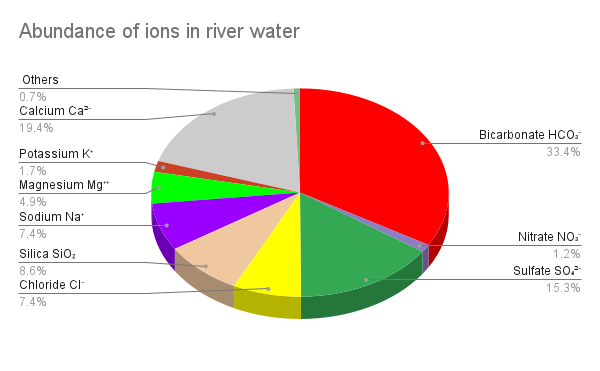
The dissolved loads of rivers are quite variable, depending on the nature of the bedrock and the environment of weathering, but here is a typical list of the nine common dissolved ions and molecules that make up 99% of the dissolved material in typical rivers[3].
- Bicarbonate HCO3– (33.4%)
- Calcium Ca2+ (19.4%)
- Sulfate SO42- (15.3 %)
- Silica SiO2 (8.6%)
- Chloride Cl– (7.4%)
- Sodium Na+ (7.4%)
- Magnesium Mg2+ (4.9%)
- Potassium K+ (1.7%)
- Nitrate NO3– (1.2%)
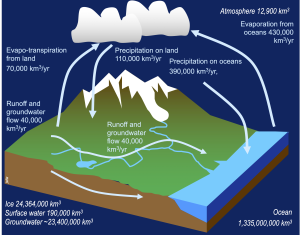
The water cycle
In an earlier section, we examined the water cycle, or hydrologic cycle, that transports water through the Atmosphere, Biosphere, and Geosphere, in adddition to the Hydrosphere. Take a short time to revisit that section, and consider where and how water has the opportunity to interact with the other spheres of the Earth.
- Although all rain is slightly acid, the term "acid rain" generally refers to water that has had its pH further lowered by additional dissolved gases such as sulfur dioxide, typically from industrial sources. ↵
- Mud is further divided at 1/256 mm, or 4 μm, into silt (coarser) and clay (finer). However, the term clay is used in several overlapping ways by Earth scientists. Clay can refer to:
- a grain-size of sediment finer than 4 μm;
- a soft sedimentary rock used for making pottery, mostly of clay grain-size, and
- a loose grouping of silicate minerals that typically form very fine crystals.
- Clarke, F.W. 1924 The Composition of the River and Lake Waters of the United States. USGS Professional Paper 135 ↵
A molecule with distinct positive and negative electric charges on either end
Weak bonds between polar molecules, seen in water for instance, where the positive poles of one molecule are drawn to the negative poles of another.
Water in its solid, crystalline form
A feature of liquid surfaces that tends to reduce the area of surface.
A positively-charged hydrogen atom that has lost its only electron; responsible for the formation of hydronium ions H3O+ in water.
A positively charged ion H3O⁺ formed by the addition of a positive hydrogen ion to a molecule of water.
Acid, acidic: having a higher concentration of hydrogen (hydronium) ions than pure water
A substance that absorbs positive hydrogen ions and/or contributes negative hydroxyl ions when dissolved in water, leading to a high pH.
Describes a substance that is able to accept hydrogen ions or generate hydroxyl ions. Alkaline.
The standard scale of a substance’s acidity or alkalinity; minus the logarithmic concentration of hydrogen ions; acids have low pH; alkalis (bases) have high pH.
A gas in which each molecule combines one atom of carbon with two of oxygen; formula: CO2
A negatively charged ion with the formula HCO3-, or a compound containing such ions
The part of a system that is separated from other parts by distinct boundary surfaces; for example water exists in three phases: solid ice, liquid, and vapour, which do not mix.
A graphical representation showing the conditions of temperature, pressure, or composition in which different phases are stable
A state wherein a compound is unlikely to undergo a phase change or chemical reaction.
A point in a phase diagram where a substance (such as water) can remain in equilibrium in the liquid, gas, and solid states simultaneously.
Water having a salinity greater than normal sea water (35 parts per thousand)
High pressure fluid that combines properties of a liquid and a gas
The line or surface separating phases in the real world, or in a "phase diagram" representing the stability conditions of different phases.
Energy released or absorbed in phase changes.
The physical breakdown of rock into smaller particles at the surface of the Geosphere.
Changes in the mineral composition of rocks as they are exposed to air and water at the Earth's surface, producing new minerals and dissolved material
Weathered material that remains in its original position, without removal by erosion. Regolith mixed with plant material is called soil.
A mixture of weathered, fragmented rock and organic matter at the surface of the Geosphere
The mechanical weathering effect of water as it freezes to form ice, due to expansion.
The mechanical weathering effect of water in thin cracks as it freezes to form ice, splitting rocks apart as a result of expansion.
The mechanical weathering effect of water trapped in spaces just below the surface of the Geosphere, raising rock or soil as it freezes to form ice.
Physical weathering caused by contact between moving rock fragments and larger rock surfaces
A feature of cliffs that have been undercut as a result of weathering and erosion, producing a hole or window through solid rock.
Upstanding masses of rock isolated from adjacent sea cliffs by erosion.
The movement of particles along the bed of a river without rotation.
Rotating movement of particles along the bed of a river.
The movement of particles in water as part of bed load, with a jumping motion. Particles are pulled briefly upward out of the bed by pressure effects, and carried downstream as they fall back to the bed.
Sediment carried along the water-bottom (usually of a river) through rolling, sliding, and saltation
Swirling movements in a fluid that developed due to the velocity of a current.
The process whereby particles remain above the bed of a body of water, because they are carried upward by fluid turbulence at a rate faster than their speed of settling.
Cloudy water caused by suspended load.
Sheet-like; applied to flow of fluids, it describes flow along sub-parallel lines, lacking the gyres typical of turblence.
Material that travels in flowing water in solution, rather than as solid particles
The continuous transport of water between the various spheres of the Earth through a series of reservoirs